
Updated Guidance for
The New York State COVID-19 Vaccination Program for Individuals 12 Years of Age or Older
June 13, 2022
Note: This guidance document applies specifically to health care providers offering COVID-19 vaccinations to adolescents and adults ages 12 and older. Guidance specific to COVID-19 vaccination of children ages 5-11 can be found on the NYS COVID-19 Vaccine Information for Providers.
Table of Contents
- Summary of Recent Changes
- Key Points about COVID-19 Vaccine for Ages 12 and Older
- Scheduling the Second COVID-19 Vaccine Dose
- Special Considerations for Receiving their Primary Series Doses Outside New York State
- COVID-19 Vaccines Listed for Emergency Use by the WHO
- Vaccine Safety
- Consent for Vaccination of Minors
- Gray Cap Pfizer Adult/Adolescent Formulation
- EUAs, FDA Vaccine Approval Status, and Appropriate Use of Vaccines in New York State
- Vaccine Management
- Equity and Access
- Communicating the Plan
- References and Resources
-
Since recommendations and eligibility for COVID-19 vaccination are expected to be updated repeatedly in coming months, and New York State (NYS) clinical guidance is aligned with CDC clinical guidance, the format of this document has been updated to refer providers to CDC’s Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States for the most up-to- date clinical guidance. NYS clinicians are encouraged to read and become thoroughly familiar with CDC’s document, which is the best source for both general guidance and guidance for special situations. This NYS guidance document retains a list of recent changes, key points, sections with NYS-specific information, sections for certain special situations, and sections or links with important information that is not included in CDC’s Clinical Considerations document (e.g., storage and handling.)
-
Update as of May 20, 2022: The following people should receive a second COVID-19 booster dose: (1) people ages 12 years and older who are moderately to severely immunocompromised, and (2) people ages 50 years and older.
- On May 20, 2022, the CDC clinical considerations were amended to provide considerations for people who recently had SARS-CoV-2 infection to delay their primary series or booster doses by 3 months from symptom onset or positive test (if infection was asymptomatic). For more information, please visit the CDC clinical considerations here.
-
Update as of May 20, 2022: the CDC has updated clinical considerations for COVID-19 re-vaccination for patients who received one or more doses of COVID-19 vaccine during treatment with B-cell depleting therapies (e.g., rituximab, ocrelizumab) that were administered over a limited period of time. More information regaining COVID-19 revaccination considerations can be found here.
- New clarifying updates were made regarding the considerations to initiate COVID-19 vaccination in individuals with a history of multisystem inflammatory syndrome (MIS-C and MIS-A). These updates can be found here.
- Based on an updated risk-benefit analysis, the FDA Emergency Use Authorization for Janssen COVID-19 vaccine has now been limited in use to specific situations. COVID-19 vaccine recipients should be informed that mRNA COVID-19 vaccines are preferred over the Janssen COVID-19 vaccine. For more information on the Janssen COVID-19 vaccines limited use, please visit the CDC’s clinical considerations here.
- There are updates to information about individuals who were vaccinated outside the U.S. and individuals who were vaccinated as part of a clinical trial. See Appendices A (People who received COVID-19 vaccine outside the United States) and B (People who received COVID-19 vaccine as part of a clinical trial) for recommendations for these populations.
Key Points About COVID-19 Vaccine for Ages 12 and Older
- All individuals 12 and older are eligible to receive an age-appropriate COVID-19 primary vaccine series; an mRNA vaccine is preferred.
- Specifically, individuals 12-17 years old are currently only eligible to receive the Pfizer-BioNTech COVID-19 vaccine primary series (i.e., purple and grey caps). Individuals 18 years and older can receive any COVID-19 primary vaccine series that is currently FDA approved or authorized in the United States (i.e., Pfizer, Moderna, or Janssen).
- Moderately to severely immunocompetent individuals are eligible for a third additional primary dose.
- The CDC endorses and recommends a clinical preference for individuals aged 18 years and older to receive an mRNA COVID-19 vaccine over the Janssen (also known as Johnson & Johnson) COVID-19 vaccine.
-
Individuals aged 5 years and older are considered up to date with their COVID-19 vaccines when they have received all doses in the primary series and all booster doses recommended for them, when eligible.
- All individuals aged 5-17 years should receive a booster dose of an FDA-approved or -authorized COVID- 19 mRNA vaccine. Individuals aged 18 years or older should receive any FDA-approved or -authorized COVID-19 vaccine as the booster dose, however, Janssen should only be used in limited situations and cannot be used as a second booster dose. Those who elect to receive the Janssen COVID-19 vaccine should be informed about the risk and symptoms of thrombosis with thrombocytopenia syndrome (TTS). For more information regarding booster dose eligibility, please visit the CDC’s clinical considerations here.
- The time intervals between the COVID-19 primary series and COVID-19 booster doses differ based on type of COVID-19 vaccine, age group, current SARS-CoV-2 infection, the vaccine recipients’ level of immunocompetency, and time since last dose administration. For more information, please visit the CDC’s Clinical Considerations here.
- For information regarding the time interval between the primary series and booster doses for individuals:
- Who are NOT moderately to severely immunocompromised, please visit the CDC’s clinicalconsiderations here.
- Who ARE moderately to severely immunocompromised, please visit the CDC’s clinical considerations here. Attempts should be made to match the additional dose type to the mRNA primary series, however if that is not feasible, a heterologous additional dose is permitted.
- There is no minimum interval between COVID-19 vaccine and other vaccines. CDC states that “COVID-19 vaccines may be administered without regard to timing of other vaccines. This includes simultaneous administration of COVID-19 vaccine and other vaccines on the same day.” For more information, see CDC’s Interim Clinical Considerations, section entitled “Coadministration of COVID-19 vaccines with other vaccines”.
Scheduling the Second COVID-19 Vaccine Dose
All providers should schedule the second dose appointment for recipients at the time the first dose is administered. If scheduling a second dose appointment is not possible, providers must supply information on how/where to obtain a second dose of vaccine.
Circumstances may arise where individuals need to receive their second dose at a different location than their first. Providers who have determined that the individual cannot return to the location where they received their first dose should either schedule a second dose for these individuals elsewhere or must supply information on how/where to obtain a second dose of vaccine. Vaccine availability can be located using the CDC’s VaccineFinder. Please ensure all individuals are informed on how to locate second dose appointment.
Special Considerations for Individuals Receiving Their Primary Series Doses within the United States but Outside New York State
Individuals who received their primary series of COVID-19 vaccine (one or both doses) outside of New York State will not have a record of this dose(s) in NYSIIS or CIR. Providers should either enter the dose(s) in NYSIIS/CIR as part of the historical record using data listed on the individual’s COVID-19 Vaccination Record Card OR advise the patient that they should ask their primary care provider to enter their primary series doses into NYSIIS/CIR so the state has a full record of both doses of COVID-19 vaccine.
Special Considerations for Individuals Receiving COVID-19 Vaccine Outside the United States
The CDC guidance for fully vaccinated people states that “this [CDC] guidance can also be applied to COVID-19 vaccines that have been authorized for emergency use by the World Health Organization (WHO) (e.g.,AstraZeneca/Oxford).”
Recommendations for people vaccinated outside of the United States depend on the number and type of vaccine(s) received for the primary series and/or booster doses. People in this scenario are considered to be up to date with their COVID-19 vaccines when they have completed vaccination as described within the CDC’s Clinical Considerations Appendix A. More information is available here.
Individuals who received the first dose of a two-dose mRNA COVID-19 vaccine are not considered fully vaccinated in the United States. They should be offered an age-appropriate second dose of an mRNA vaccine (i.e., Pfizer-BioNTech COVID-19 Vaccine).
For COVID-19 vaccines not authorized by the FDA but listed for emergency use by the WHO:
- Please visit the CDC’s guidance on vaccines listed for emergency use by the WHO but notapproved/authorized by the FDA.
- Individuals who have received all recommended doses of a COVID-19 vaccine that is listed for emergency use by the WHO do not need any additional doses with an FDA-authorized COVID-19 vaccine.For COVID-19 vaccines neither authorized by FDA nor listed for emergency use by the WHO:
- For individuals who received all or some of the recommended doses of a COVID-19 vaccine that is neither authorized by FDA nor listed for emergency use by the WHO, the CDC does NOT consider these individuals to be fully vaccinated. They should be offered a two-dose series of the age-appropriate COVID-19 vaccine (i.e., Pfizer-BioNTech or Moderna COVID-19 Vaccine formulation). For more information, please visit the CDC’s guidance on these vaccines here.
COVID-19 Vaccines Listed for Emergency Use by the WHO
As of May 1, 2022, the WHO has listed a number of COVID-19 vaccines for emergency use. There are several vaccines on this list that are also authorized by the FDA for Emergency Use in the United States. These include:
- Pfizer-BioNTech COVID-19 vaccines (e.g., COMIRNATY, Tozinameran)
- Janssen (Johnson & Johnson) COVID-19 vaccine
- Moderna COVID-19 vaccine (Spikevax)
For more information regarding other vaccines listed for emergency use by the WHO, please visit the WHO website. Please note that the minimum interval between receipt of the non-FDA-approved/authorized vaccine and initiation of the FDA-approved/authorized COVID-19 vaccine primary series is at least 28 days.
Post-vaccination monitoring is an essential part of the COVID-19 vaccination program. The CDC is promoting and encouraging all those being vaccinated to participate in V-Safe, a smart-phone based application that will allow those vaccinated to enter their symptoms in the days after vaccination using text messaging. V-Safe also provides reminders for the second dose and telephone follow up for anyone who reports medically significant adverse events. V-Safe materials can be found at http://www.cdc.gov/vsafe, including a V-Safe information sheet. Please print out the information sheet and hand to each person vaccinated.
You must report any adverse events that occur after vaccination to the Vaccine Adverse Events Reporting System (VAERS) at [email protected]g or by calling 1-800-822-7967. For a list of administration errors and deviations and what action to take after an error or deviation has occurred, please refer to this CDC resource: Appendix C. Vaccine Administration Errors and Deviations.
Information on COVID19 vaccine safety signals that have been assessed by one or more of these mechanisms can be found in CDC’s Selected Adverse Events Reported after COVID-19 Vaccination. Additional information can be found in CDC’s Interim Clinical Considerations:
-
Section entitled Safety considerations for mRNA COVID-19 vaccines: Pfizer-BioNTech and Moderna (including considerations surrounding myocarditis and pericarditis), and
-
Section entitled COVID-19 vaccination and SARS-CoV-2 infection including MIS-C and MIS-A (including considerations for vaccination after MIS-C).
Serious safety problems associated with COVID-19 vaccines are rare. Still, patient perception of COVID19 vaccine safety, often fueled by false reports on social media, can affect public trust in vaccination. Information on common myths about COVID19 vaccine safety (including false information on the vaccines’ effects on fertility and DNA) can be found at the CDC’s Facts webpage and New York State’s webpage Combatting Misinformation About the COVID-19 Vaccines.
Consent for Vaccination of Minors
Entities operating vaccination sites may use the following verification methods as a model for securing consent for vaccination of minors, in consultation with counsel as needed. It is important to verify the age of any individual who appears to be a minor to ensure consent is obtained, confirm eligibility, and ensure the administration of the proper COVID-19 vaccine.
Proof of age should be requested but is not required where the parent or guardian is available to attest to the
minor’s age. Documentary proof may include (but is not limited to):
- Driver’s license or non-driver ID
- Birth certificate issued by a state or local government
- Consulate ID
- Current US passport or valid foreign passport
- Permanent resident card
- Certificate of Naturalization or Citizenship
- Life insurance policy with birthdate
- Parent/guardian attestation
For all minors, a parent or legal guardian must provide consent for vaccination.
16 and 17-year-olds:
For minors 16 or 17 years of age, consent should be provided either in person or by phone, at the time of vaccine appointment. Providers may elect to accept a written statement of consent from the parent or guardian, where the parent or guardian is not available by phone to provide consent to vaccinate an unaccompanied minor.
5 through 15-year-olds:
For minors who are 5 through 15 years of age, additionally, an adult caregiver should accompany the minor. If the adult caregiver is not the parent/guardian, the adult caregiver should be designated by the parent/guardian. The parent/guardian must still provide consent to the vaccination.
“Gray Cap” Pfizer Adult/Adolescent Formulation:
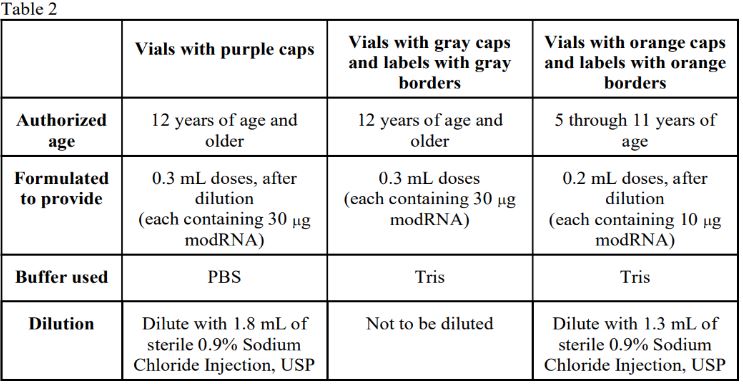
On December 23, 2021 a new formulation of Pfizer adult/adolescent vaccine was introduced. This formulation is being referred to as Pfizer Adult/Adolescent Tris (Gray Cap, age 12+, no diluent). This formulation contains the Tris Sucrose buffer, does not require dilution at administration sites, may be stored at refrigerated temperature (2-8° C) for up to 10 weeks, and can be used on individuals 12 years of age and older. The vials will have a gray cap and label with gray border. Individuals who received a previous dose or doses of the Pfizer 12+ “purple cap” vials may receive the Pfizer Adult/Adolescent Tris (Gray Cap, age 12+, no diluent) formulation for primary, additional, or booster doses.
Pfizer Adult/Adolescent Tris (Gray Cap, age 12+, no diluent) is a multiple dose vial that contains a volume of 2.25 mL and is supplied as a frozen suspension that does not contain preservative. Each vial must be thawed prior to administration. DO NOT DILUTE prior to use. One vial contains 6 doses of 0.3 mL. Even though the vaccine does not require dilution, the vial must be mixed by gently inverting the vial 10 times before puncture.
Vaccination providers should maximize using inventory of all Pfizer 12+ purple cap vials prior to using Pfizer Adult/Adolescent Tris (Gray Cap, age 12+, no diluent) vials and should ideally carry only one Pfizer adult formulation at a time. The shipping containers used for this formulation are single use and cannot be used for temporary storage with dry ice or for vaccine transport. To avoid mistakes during this period of transition when both adult formulation products may be in circulation, these products should not be offered/administered at the same time. *See Table 2 below.
The Pfizer-BioNTech COVID-19 vaccine, supplied in two formulations, is provided in three different color-coded multiple dose vials:
EUAs, FDA Vaccine Approval Status, and Appropriate Use of Vaccines in New York State
Providers must administer COVID-19 vaccines in accordance with all program requirements and recommendations of NYSDOH and the CDC, the Advisory Committee on Immunization Practices, and the U.S Food and Drug Administration (FDA). This applies to vaccines administered in accordance with an EUA or Emergency Use Instruction (EUI), as well as FDA approved COVID-19 vaccines. Accordingly, use of these products outside of those that have been approved and authorized by FDA or in accordance with a CDC EUI (often referred to as “off-label use”) is not recommended. It would violate the provider agreement and could expose providers to the following risks:
- Administration of the product off label may not be covered under the Public Readiness and Emergency Preparedness (PREP) Act or the PREP Act declaration; therefore, providers may not have immunity from claims.
- Individuals who receive an off-label dose may not be eligible for compensation under the Countermeasures Injury Compensation Program after a possible adverse event.
- CDC has defined the scope of the CDC COVID-19 Vaccination Program in terms of how the USG-provided vaccines may be used in the program. Providers giving off-label doses would be in violation of the CDC Program provider agreement potentially impacting their ability to remain a provider in the CDC program.Administration fees may not be reimbursable by payers.Accurate and timely reporting to NYSIIS/CIR is critical, as this information can be used to allow individuals to display proof of vaccination, such as the Excelsior Pass or Excelsior Pass Plus.
Please see the NYSDOH COVID-19 Vaccine Information for Providers page for more information on ordering pediatric Pfizer-BioNTech COVID-19 vaccine in NYSIIS. Providers in New York City should follow instructions from NYC DOHMH and CIR.
All facilities or practices are required to track vaccine uptake among their staff and must furnish uptake data to the NYSDOH via HERDS survey upon request, or as directed by your agency or organization.
Storage and Handling Requirements
Vaccines must be stored and handled properly from the time they are manufactured until they are administered to maintain the cold chain, thus protecting the potency and effectiveness of the vaccine and ensuring vaccine recipients are fully and safely protected from vaccine-preventable diseases. Detailed information regarding COVID-19 Vaccine storage and handling requirements can be found at CDC Vaccine Storage and Handling Toolkit. A storage and handling summary for each product can be found on the CDC COVID-19 Vaccine: Quick Reference Guide for Healthcare Providers.
As part of the COVID-19 Vaccination Provider Agreement, providers are required to:
- Store and handle vaccines under proper conditions, including maintaining cold chain conditions and chain of custody at all times in accordance with an EUA or vaccine package insert, manufacturer guidance, and CDC guidance in the Vaccine Storage and Handling Toolkit.
- Monitor storage unit temperatures at all times, using equipment and practices that comply with guidance in the toolkit. Every storage unit that holds COVID-19 vaccines must have a digital data logger (DDL). Staff must check and record temperatures each workday and regularly check the DDL temperature data.
- If the temperature of the storage unit goes outside of the recommended temperature range, the temperature excursion must be reported immediately. Providers located outside NYC must complete the COVID-19 Vaccination Program Temperature Excursion Report.
- Monitor and comply with COVID-19 vaccine expiration and beyond use dates (see sections below).
- Preserve all records related to COVID-19 vaccine management, including temperature records, for a minimum of three years.
- Comply with CDC instructions and timelines for disposing of COVID-19 vaccine and diluent, including unused doses.
COVID-19 Vaccine Expiration and Beyond Use Dates
Expiration Dates:
Determining when a vaccine expires is a critical step in proper storage and handling. The expiration date should always be checked prior to preparing or administering vaccine. Expired vaccine or diluent should NEVER be used. As additional stability data become available, the expiration dates for some products may change. Expiration dates for Pfizer products can be found here. Follow the instructions below to determine the expiration date:
-
Pfizer-BioNTech COVID-19 vaccine for ages 12 and older (vials have purple caps): FDA approved an amendment to the EUA for Pfizer-BioNTech COVID-19 vaccine extending the expiration dates of purple cap vials to 12 months. Cartons and vials of Pfizer-BioNTech COVID-19 vaccine with an expiry date of November 2021 through March 2022 printed on the label may remain in use for 6 months beyond the printed date as long as authorized storage conditions between -90°C to -60°C (-130°F to -76°F) have been maintained. Frozen vials stored at -25°C to -15°C and refrigerated vials (2°C to 8°C) are NOT eligible for extension. Updated expiry dates for vaccine maintained in ultra-cold storage are shown below. The extended expiration date is effective immediately for all currently available batches that have not yet expired. Vaccine cannot be used after the new expiration date, even if the storage-determined beyond- use date would be after the updated expiration date.
Vaccine cannot be used after the new expiration date, even if the storage-determined beyond-use date would be after the updated expiration date.
Printed Expiry Date
Updated Expiry Date
November 2021
May 31, 2022
December 2021
June 20, 2022
January 2022
July 31, 2022
February 2022
August 31, 2022
March 2022
September 30, 2022
- Pfizer Adult/Adolescent Tris (Gray Cap, age 12+, no diluent): The date printed on the Pfizer Adult/Adolescent Tris (Gray Cap, age 12+, no diluent) vaccine vials indicate the manufacture date and NOT the expiration date. Originally, the expiration date was 6 months from the manufacture date, when stored in ultra-cold freezer temperatures (-90 to -60° C). The expiration date for Pfizer gray cap vaccine has now been extended to 12 months (while held at ULT frozen.) Vials may also be stored up to 10 weeks in the refrigerator (2-8° C). No standard freezer storage is approved for the Pfizer Adult/Adolescent Tris (Gray Cap, age 12+, no diluent) formulation. Once thawed, vials CANNOT be refrozen.
The Fact Sheet for the Pfizer Adult/Adolescent Tris (Gray Cap, age 12+, no diluent) provided by the FDA now reads “regardless of storage conditions, vaccines should not be used after 12 months from the date of manufacture printed on the vial and cartons.” Therefore, vaccine must be used by the expiration date, or the 10-week beyond use date for refrigerator storage, whichever comes first. The updated expiry dates for the gray cap vials based on 12 months from the date of manufacture are provided below:
Printed Manufacturing Date
12-Month Expiry Date*
06/2021
May 31, 2022
07/2021
June 30, 2022
08/2021
July 31, 2022
09/2021
August 31, 2022
10/2021
September 30, 2022
11/2021
October 31, 2022
12/2021
November 30, 2022
01/2022
December 31, 2022
02/2022
January 31, 2022
*Date of expiration always falls on the last day of the month
- Moderna COVID-19 vaccine: The expiration date is NOT printed on the vaccine vial or carton.
-
To obtain the expiration date of the lot number received, providers can scan the QR code located on the vial or carton or access the manufacturer’s website directly, enter the lot number and the expiration date will be displayed.
- In September 2021, Moderna submitted data to support the extension of certain lot number expiration dates. Prior to discarding expired lots of Moderna vaccine, it is important to re-check the manufacturer’s website to determine if the lot number’s expiration date has been extended.If an extension was made, providers need to ensure the expiration date on the vials/packages and in NYSIIS/CIR are updated.
-
- Janssen/Johnson & Johnson COVID-19 vaccine: The expiration date is NOT printed on the vaccine vial or carton.
- On April 27, 2022, the FDA announced the approval of another shelf-life extension for refrigerated Janssen vaccine. This decision is based on data from ongoing stability assessment studies, which have demonstrated that the vaccine is now stable at 11 months when refrigerated at temperatures of 36o – 46o Fahrenheit (2o – 8o Celsius).
- To determine the most current expiration date:
- Scan the QR code located on the outer carton, or
- Call 1-800-565-4008, or
- Go to https://vaxcheck.jnj/, enter the lot number and the expiration date will be displayed.For Moderna and Janssen/J&J COVID-19 vaccines it is important to write the expiration date on the carton or vials since it is not printed. Orders of Moderna and Janssen/J&J received in NYSIIS or CIR will contain a placeholder date of 12/31/2069. The actual expiration date must be updated in NYSIIS or CIR, as well as part of inventory management. Vaccines should always follow a first in, first out process in which vials that have theearliest expiration date are used first. CDC’s https://www.cdc.gov/vaccines/covid-19/downloads/expiration- tracker.pdf can help providers keep track of the expiration date by lot number. Vaccine inventory should be managed using a “first in first out” tracking process to limit the potential for wastage.
Beyond Use Dates (BUDs):
All vaccines have expiration dates, and some routinely recommended vaccines have a beyond use date (BUD), which is calculated based on the date the vial is first punctured and the storage information in the package insert. Whenever a vial of COVID-19 vaccine is moved to storage conditions that affect BUD or a multidose vial is punctured, label the vial(s) with the beyond use date/time. The BUD must never exceed the labeled expiration date. Once the vaccine has reached its expiration or beyond use date/time, unused doses must be disposed of as medical waste and reported as wastage in NYSIIS or CIR. A summary of COVID-19 vaccine beyond use dates and resources are listed below:
- Pfizer age 12 and older (vials have purple caps): Pfizer-BioNTech COVID-19 Vaccine Beyond-Use Date (BUD) Tracking Labels for Vaccine During Freezer or Refrigerator Storage
-
Freezer (-25° C to -15° C): Two weeks
-
Refrigerator (2° C to 8° C): 31 days
-
After Puncture: 2° C to 25° C for up to 6 hours
-
- Pfizer Adult/Adolescent Tris (Gray Cap, age 12+, no diluent): Beyond-Use Date (BUD) Tracking Labels for Vaccine During Refrigerator Storage
-
Refrigerator (2° C to 8° C): 10 weeks
-
NOTE: NO standard freezer (-25° C to -15° C) storage allowed
-
Room temperature (8 ° C to 25° C): 12 hours prior to first puncture
-
After Puncture: 2° C to 25° C for up to 12 hours. Vial labels and cartons may state that a vial should be discarded 6 hours after the first puncture. The information in the EUA Fact Sheet (12 hours) supersedes the number of hours printed on vial labels and cartons.
-
- Moderna: Moderna COVID-19 Vaccine Beyond-Use Date (BUD) Tracking Label for Vaccine During Refrigerator Storage
-
Refrigerator (2° C to 8° C): 30 days
-
After Puncture: 2° C to 25° C for up to 12 hours
-
- Janssen/J&J: Janssen COVID-19 Vaccine Preparation and Administration Summary
- ONLY store in refrigerator up to expiration date.
-
After Puncture: 2° C to 8° C up to 6 hours OR 9° C to 25° C for up to 2 hours. These times are NOT cumulative (i.e., you cannot store a punctured vial for 6 hours at refrigerated temperatures and then another 2 hours at room temperature).
Moderna Booster Dose Inventory Considerations
It is important to note that the volume of a Moderna booster dose is 0.25 mL (half the volume of a primary dose). The Moderna COVID-19 vaccine was previously supplied in two multiple-dose vial presentations. The 14- dose vials (NDC 80777-0273-98) have all expired and should no longer be in use. The only vials of Moderna currently in distribution in the United States are multiple-dose vials containing 5.5 mL (i.e., Moderna 10-dose).
Reporting: Despite the volume of the booster dose being 0.25 mL, providers must still report a full dose as administered in NYSIIS. Reporting of half doses is not allowed and inventory must only be reported in whole doses. Half doses in NYSIIS inventory will prevent a provider from entering new vaccine orders.
Maximum vial puncture: Providers may extract both primary series doses (0.5mL) and booster doses (0.25 mL) from the same vial. When extracting only booster doses or a combination of primary series and booster doses, the maximum number of doses that may be extracted from either vial presentation should not exceed 20 doses. Do not puncture the vial stopper more than 20 times.
- After the vial has been punctured 20 times, the vial must be discarded, even if there is vaccine remaining in the vial and the beyond use date/time has not been reached (see more info below on when to report wastage in NYSIIS).
- The use of vial adapters, dispensing pins, or strategies where a needle is inserted into the vial septum for multiple medication withdraws is not allowed due to contamination risk.
NYSIIS inventory: Due to the reporting of full doses for boosters and the maximum of 20 punctures for each vial, the number of doses reported may exceed the number of doses recorded in NYSIIS inventory (i.e., 100 dose order = up to 200 booster doses). This means NYSIIS inventory may be depleted before physical inventory. Best practice would be to modify inventory to add doses to the lot number BEFORE ADMINISTRATION. Do a vial count of physical inventory at the end of the day and multiply your full, unopened vials times the number of labeled doses in the vial (10 doses) and manually modify your NYSIIS inventory to reflect this count. If you report vaccine administration data via data exchange, additional doses beyond the NYSIIS doses on hand will go to the Inventory Not Deducted module. If this happens, manually add doses to the lot number and then update non- deducted inventory.
NYSIIS inventory is used to populate Vaccine Finder product availability through a daily data upload. If you have physical inventory and you do not modify inventory to add doses once it is depleted in NYSIIS, your location will not show as having Moderna vaccine available on Vaccine Finder.
Wastage: Continue to maintain reporting of wastage in whole doses. Wastage should only be reported if the total doses administered from a vial, regardless of volume or series, is less than the vial dose count (i.e., 1
primary and 5 booster doses from a 10-dose vial would be reported as 6 doses used and 2 doses wasted). Once 10 doses are given from a 10-dose vial, regardless of whether primary or booster doses, no wastage needs to be reported even if there is vaccine remaining in the vial. Post-puncture times must still be tracked and remaining doses discarded as medical waste.
As the ordering quantities and the storage conditions have become more practical, providers are encouraged to place direct orders in NYSIIS and avoid redistribution whenever possible, even if all doses cannot be used.
Vaccine may be redistributed to another facility, provider, practice, or local health department that is enrolled in the COVID-19 vaccination program, with proper notice to the NYSDOH. Prior to redistributing vaccine, facilities must submit a completed redistribution form to [email protected] and can proceed with the redistribution once submitted. Redistributions must follow the New York State COVID-19 Vaccine Program Guidance for Vaccine Transport, including use of a digital data logger to monitor temperatures during transport. Direct orders are the preferred and safest way to receive vaccine.
A provider may transport vaccine to another location for the purpose of holding a limited duration vaccination clinic without notifying the NYSDOH. If the provider is administering the doses and reporting doses administered against their own inventory in NYSIIS, all unused vaccine must be transported back to the original location at the conclusion of the clinic that day. The provider must retain possession and control of the vaccine for the duration of the transport and administration. All transports, whether for off-site clinics or redistribution, require adherence to the COVID-19 Transport Guidance the completion of the COVID-19 Transport Tracking Sheet (found on page 7 of the guidance).
The CDC released guidance on May 11, 2021, regarding wastage with the critical message to “take every opportunity to vaccinate every eligible person.” As more vaccination opportunities are created, the likelihood of leaving unused doses in a vial may increase. While enrolled providers must continue to follow best practices to follow clinical best practices to use every dose possible, it should not be at the expense of missing an opportunity to vaccinate every eligible person when they are ready to get vaccinated.
As the ordering quantities and the storage conditions have become more practical, we are encouraging providers to place direct orders whenever possible, even if you cannot use all doses. This is the safest way for providers to receive vaccine and reduces the risk of temperature excursions and the burden of continued
redistribution.
-
Currently available COVID-19 vaccine products are all multidose vials. Vaccine vials must often be punctured without using the full number of doses printed on the label. Do not turn anyone away because you do not have additional people to vaccinate with remaining doses in a vial. Discarding the remaining doses is acceptable wastage (and needs to be reported as wastage in NYSIIS or CIR). Doses not administered within the limits below post-puncture must be wasted:
- 12 hours: Moderna and Pfizer Adult/Adolescent Tris (Gray Cap, age 12+, no diluent)
- 6 hours: Pfizer-BioNTech 12+ purple cap vials
- 6 hours (refrigerated) or up to 2 hours at room temperature: J&J/Janssen. These times are NOT cumulative (i.e., you cannot store a punctured vial for 6 hours at refrigerated temperatures and then another 2 hours at room temperature).
Effort must be made to do outreach to individuals 12 years of age and older in all communities and settings. Individuals in areas that have a high social vulnerability index are particularly vulnerable to COVID-19 and should be notified about how they can receive vaccine. Every effort should be made to increase their access to vaccination opportunities.
Please be sure to clearly communicate this critical guidance to all staff involved in the vaccination program.
This guidance is in effect from the date of issuance until it is updated, or additional guidance is issued by NYSDOH. For questions, please contact the New York State Department of Health, Bureau of Immunization at [email protected].
- At-A-Glance COVID-19 Vaccination Schedules
- Pfizer-BioNTech COVID-19 Vaccine (Purple Cap, Must Dilute) FDA EUA for 12 Years of Age and Older for Healthcare Providers
- Pfizer-BioNTech COVID-19 Vaccine (Grey Cap, No Dilution) FDA EUA for 12 Years of Age and Older for Healthcare Providers
- Pfizer-BioNTech COVID-19 Vaccine FDA EUA (12 Years of Age and Older) for Caregivers and Recipients
- Moderna COVID-19 Vaccine FDA EUA for Caregivers and Recipients
- Moderna COVID-19 Vaccine FDA EUA for Vaccination Providers for Primary Series and Booster Dose
- Janssen COVID-19 Vaccine FDA EUA for Caregivers and Recipients
- Janssen COVID-19 Vaccine FDA EUA for Vaccination Providers
- Interim recommendations for COVID-19 vaccine administration errors and deviations
- Medical Management of Vaccine Reactions in Children and Teens in a Community Setting (Immunization Action Coalition)
- Protective Measures for Vaccinating During the COVID-19 Pandemic (Immunization Action Coalition)
- Skills Checklist for Vaccine Administration (Immunization Action Coalition)
- Supplies You May Need at an Immunization Clinic (Immunization Action Coalition)
- Ask the Experts: COVID-19 Specific Information (Immunization Action Coalition)
- Ask the Experts: Administering Vaccines (Immunization Action Coalition)
- Additional information about the level of immune suppression associated with a range of medical conditions and treatments can be found in general best practices for vaccination of people with altered immunocompetence, the CDC Yellow Book, and the Infectious Diseases Society of America policy statement, 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host.
Share
FDA Authorizes Updated (Bivalent) COVID-19 Vaccines for Children Down to 6 Months of Age – December 9, 2022 Dear COVID-19 Vaccination Provider: On December 8, 2022, FDA authorized the bivalent Moderna COVID-19 vaccine for children ages 6 months through 5 years of age and bivalent Pfizer-BioNTech COVID-19 vaccine for children 6 months through 4
Yesterday, the U.S. Food and Drug Administration amended the emergency use authorizations (EUAs) of the Moderna COVID-19 Vaccine, Bivalent and the Pfizer-BioNTech COVID-19 Vaccine, Bivalent to authorize their use as a single booster dose in younger age groups: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-and-pfizer-biontech-bivalent-covid-19-vaccines. CDC’s Director Dr. Rochelle Walensky released a statement and signed a decision memo recommending updated (bivalent) COVID-19 boosters for children five years and older. This expands on CDC's recommendation issued September 1, 2022, for updated COVID-19 boosters of people ages 12 and older.
Updated Clinical Guidance For Individuals 6 Months-11 Years Old – June 22, 2022 Dear NYS COVID-19 Vaccine Provider: Effective June 18, 2022, the CDC has recommended that everyone ages 6 months and older in the United States should receive a COVID-19 primary series vaccination for the prevention of COVID-19. This attached provider guidance has
Dear NYS COVID-19 Vaccine Provider: On January 7, 2022, the CDC updated the recommendation for receipt of a booster dose of the COVID-19 vaccine following receipt of the Moderna primary series. CDC now recommends receipt of a booster dose at least five months after completing their Moderna primary series. This previously recommended interval was at

